The Woolcock Institute of Medical Research
OSA treatment may reduce diabetes and heart disease
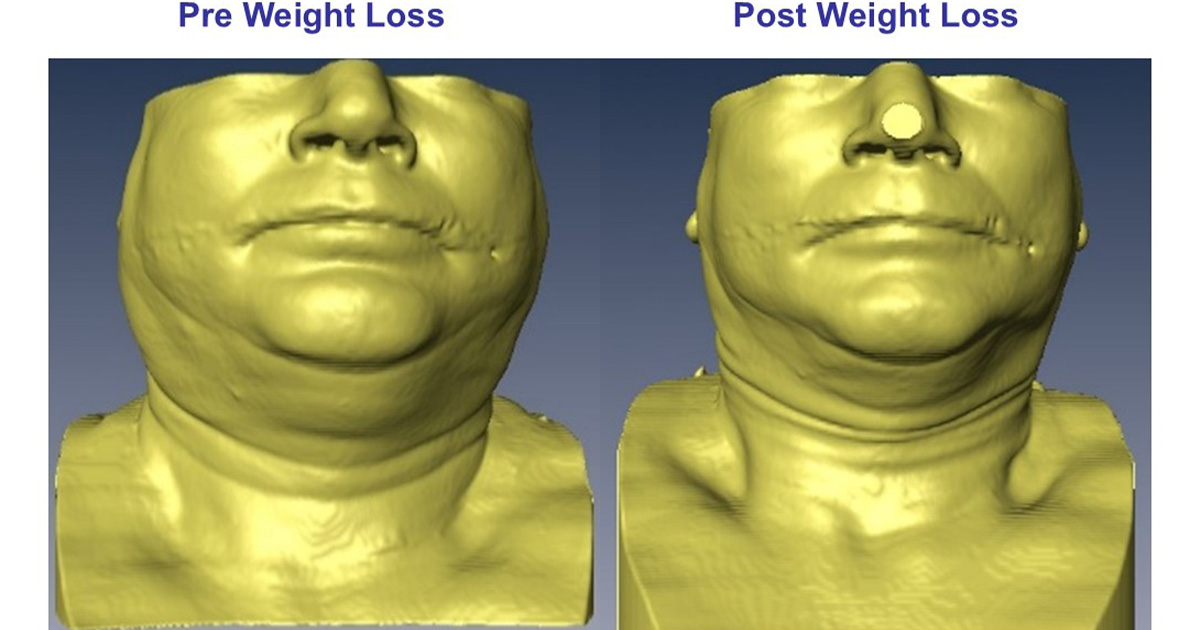
A global first study shows weight loss drug tirzepatide potentially reduces the risk of diabetes and heart disease by treating both obstructive sleep apnea (OSA) and obesity.
Conventional treatments for sleep apnea such as continuous positive airway pressure do not appear to reduce the risk of diabetes and cardiovascular disease.
The study titled “Tirzepatide on obstructive sleep-related cardiometabolic risk: secondary outcomes of the SURMOUNT-OSA randomized trial” and published in Nature Medicine evaluated the effect of the medication on 469 overweight or obese participants suffering from severe sleep apnea.
Obstructive sleep apnea, a condition where breathing repeatedly stops and starts during sleep, affects about 38% of Australians and is strongly linked to obesity and a higher risk of cardiovascular diseases like heart attack and stroke.
Building on the original research which showed the drug reduced the severity of sleep apnea with participants shedding a fifth of their weight, it further highlights the potential of the drug tirzepatide in reducing markers of cardiometabolic risks in affected people.
Want to stay up to date with our research on sleep and respiratory conditions?
Sign up to our quarterly newsletter
The global, phase-3 clinical trial took place across 60 sites in nine different countries, including Australia, and was carried out in Sydney by the Woolcock Institute of Medical Research’s sleep trials team, headed by Professor Ron Grunstein.
The research showed that participants with OSA and obesity who received tirzepatide experienced a marked reduction in various cardiometabolic risk factors compared to those on a placebo. Variables measured included markers of inflammation, pre-diabetes, triglycerides and “bad” cholesterol.
Importantly, the study investigated how these improvements occurred with tirzepatide by analysing the impact of actual weight loss alone, reduction in sleep apnea alone and the combination of these two. While weight loss alone had a major impact, the benefits of combined weight loss and better sleep apnea control using tirzepatide yielded even better results.
"This study provides compelling evidence that a holistic approach is vital for patients struggling with both obstructive sleep apnea and obesity," said Dr. Ronald Grunstein, a world-leading expert in sleep medicine at the Woolcock Institute of Medical Research and a key author on the paper.
"We’ve seen that tirzepatide can address several risk factors and is revolutionising the treatment of sleep apnea, but for the optimal benefit to heart health, tackling both the sleep-disordered breathing and the excess weight is likely essential. This opens up exciting new avenues for treatment strategies and clinical services that could significantly reduce the burden of cardiovascular disease in this vulnerable patient group."
The findings underscore the interconnectedness of sleep health and metabolic health, pointing towards future treatment paradigms that address these conditions.
The SURMOUNT-OSA trial is registered under ClinicalTrials.gov (NCT05412004) and was funded by Eli Lilly.










